The tremendous advances in technology over the past few decades allow scientists to collect and analyze much more information than they could in the past. In biology, this means researchers can do things a little differently. Where biologists used to look at fairly sparse data and make general descriptive statements, they can now combine the results of measurements with sophisticated theoretical analysis, uncovering new hypotheses and quantitatively testing them. That’s the rationale behind the Simons Foundation’s Mathematical Modeling of Living Systems (MMLS) program: to use theoretical and mathematical concepts to develop a quantitative understanding of all aspects of life. To this end, the foundation supports individual researchers through its Investigators in MMLS program, and funds targeted grants to research groups studying particular projects. The foundation also organizes an annual Conference on Theory and Biology, providing a venue for scientists in the greater New York area to meet, exchange insights and hear talks by leading scientists in the field.

Ned Wingreen of Princeton University delivers “Keeping It Together: Organizing the Bacterial Chromosome for Division” at the MMLS Theory and Biology meeting in April 2014.
Olga Zhaxybayeva is assistant professor of biological sciences at Dartmouth College, and a Simons Investigator. She sifts through bacterial genomes in an effort to understand their evolution and speciation. Modeling and data analysis go hand in hand in her work. Her group uses sophisticated mathematical models to understand how different traits and conditions influence bacterial populations, and they analyze genome databases to inform those models.
One of the focuses of Zhaxybayeva’s research group is to study the history and distribution of genes in microbial communities to determine which ones might effectively differentiate between bacterial species. “In the eukaryotic world, Linnaeus created this beautiful taxonomy where every organism is classified from species to genera to larger groups,” she says. “In bacteria, we’d like to think that organisms have some features that allow us to put them into categories that we can call species.”
However, unlike plants and animals, bacteria propagate asexually, so the ‘can produce fertile offspring’ definition of species doesn’t work. The obvious choice is to consider genetic similarity instead. Back when there were only three Escherichia coli genomes available for study, researchers compared them to determine which traits were present in all three. “We think of them as the same E. coli, but really they had only 40 percent of their genes in common,” says Zhaxybayeva. “When the study was expanded to about 60 genomes, it turned out that they only shared on the order of 5 percent of their DNA. Now that there are thousands of genomes, it’s leveled off, but it’s a very small fraction of genes.” That means it’s difficult to find genes that can effectively categorize the organisms.
Zhaxybayeva’s investigations also include research into the evolution of two bacterial traits: tolerance of extreme temperatures and what she describes as cooperation in microbial communities.
The modus operandi of many common viruses is simple: Infect a host, make copies of virus DNA or RNA, kill the host, repeat. But some bacterial populations contain entities called gene transfer agents that behave like viruses but contain bacterial genes instead of their own genome. Like viruses, they kill their hosts, but for some reason, the population continues to host the gene transfer agents. “The hypothesis is that it’s some sort of form of cooperation in bacterial populations,” says Zhaxybayeva. The question is how the gene transfer agents persist in the population. Researchers think that the bacterial DNA they contain could help ‘patch’ DNA damaged by ultraviolet radiation or other stressors, or that it could enable the exchange of beneficial genes within the population. “The bacteria population designates a small fraction of cells to ‘sacrifice’ themselves to produce gene transfer agents,” she says.
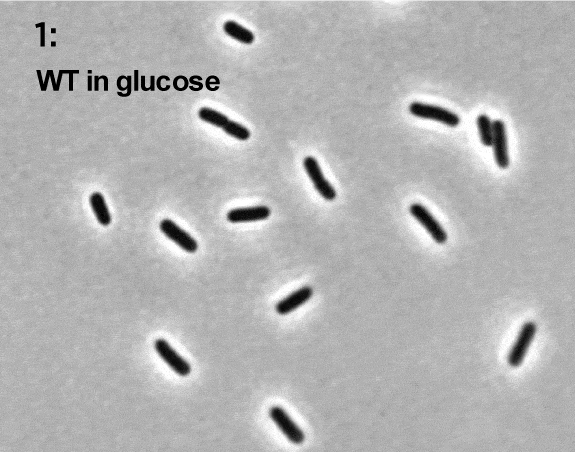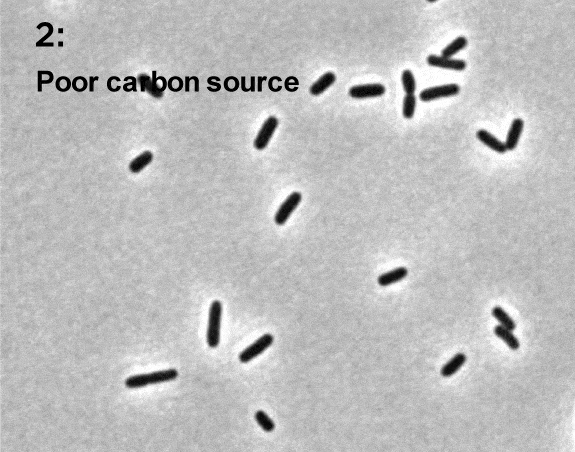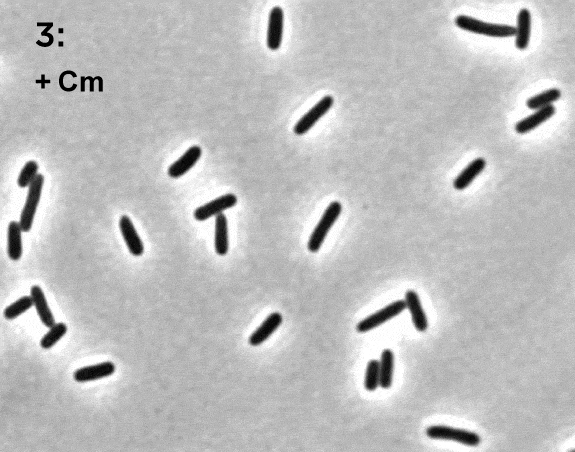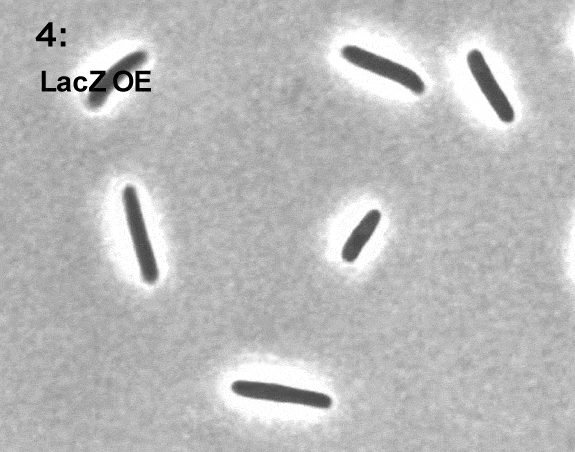
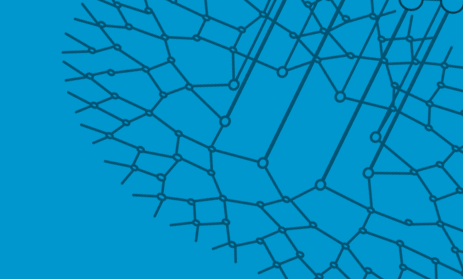
Terry Hwa’s laboratory analyzes snapshots of bacteria growing in different environments.
She and her colleagues model bacterial populations with gene transfer agents and determine how they might maintain the trait, given that it’s quite detrimental to individual hosts. On the data-analysis side, they find organisms with particular traits and use sequence comparison techniques to reconstruct the traits’ evolutionary histories. While Zhaxybayeva doesn’t do experiments herself, she collaborates with labs that grow bacteria that produce gene transfer agents, to study them on a molecular level. Both that information and her analysis of genome databases then inform later simulations and modeling.
Terry Hwa, professor of physics and biology at the University of California, San Diego, is the recipient of a targeted grant in the MMLS program. Whereas Zhaxybayeva’s doctorate is in molecular biology, Hwa’s is in theoretical physics. As a physicist, he was most interested in complexity: nonlinear dynamics, turbulence, spin glass, and so on. Over the course of his career, he started moving more toward biology. “You can’t close your eyes to the amazing complexity of life,” he says. “I was intrigued by living systems and started to poke into it. One thing led to another … ” Today he heads a lab that employs physicists, biologists and applied mathematicians to study the way interactions between molecules in a cell determine the cell’s overall behavior.
Earlier in his career, Hwa showed that organisms’ responses to environmental and genetic perturbations tend to obey simple mathematical rules, a counterintuitive finding, given how many components a cell has and how complex intracellular interactions are known to be. For example, the number of proteins a cell devotes to nutrient uptake has a negative relationship to the cell’s growth rate: The poorer the nutrients, the more uptake proteins are used to retrieve them. “We simplify the description of bacteria to ‘a bag of proteins and other small molecules,’” Hwa says. “We then see simple patterns in the macroscopic cellular behavior in selective environments, and can use these patterns to predict behaviors in other environments. Ultimately, these simple behaviors come from the interaction of molecules. Something takes us from the complexity at molecular scale to the simplicity at cellular scale.” Hwa is trying to find that something.
Hwa sees a parallel between today’s research into the molecular foundations of cellular behavior and the 19th century development of thermodynamics and statistical mechanics. At the time, the ideal gas laws and features of phase transitions were known, but they hadn’t been understood in quantitative and molecular terms. Major breakthroughs came when scientists discovered how the behavior of a gas can be described by a number of simple mathematical rules — the laws of thermodynamics — and subsequently how these laws arise mathematically from averaging over many complex molecular collisions. Hwa thinks research such as his that bridges the gap between biology and physics might lead to a similar breakthrough in our understanding of behaviors of cells on a molecular level.
As with Zhaxybayeva, real data inform Hwa’s models. His lab grows cells in different environments and takes ‘snapshots’ of their contents at various points in time. Those snapshots are analyzed to determine how much of different molecules has been produced by the cells or how the cells have grown. The data give Hwa’s team a starting point for creating their models, and later give them a way to see whether their models are on track.
“The ultimate goal is to make our model predictive,” says Hwa. “We’ve had some success at the cellular level — we want to make it predictive at the molecular level.”